Have you ever wondered about the medicines we use, and maybe, just maybe, asked yourself, "Why was Rocephin discontinued?" It's a fair question, really. People often think about why certain things change or disappear from common use, especially when it comes to something as important as a medication. This kind of curiosity, in a way, helps us understand the world around us a bit better.
There are many reasons why a drug might leave the market, or why people might get the idea that it has. Sometimes, it's about new scientific findings, or maybe a different medicine comes along that works even better. Other times, it's simply a misunderstanding, a bit like how people sometimes mix up words that sound similar, as I often hear people use the word 'jive' when I'm pretty sure they mean 'jibe'. So, let's clear up some of that confusion about Rocephin.
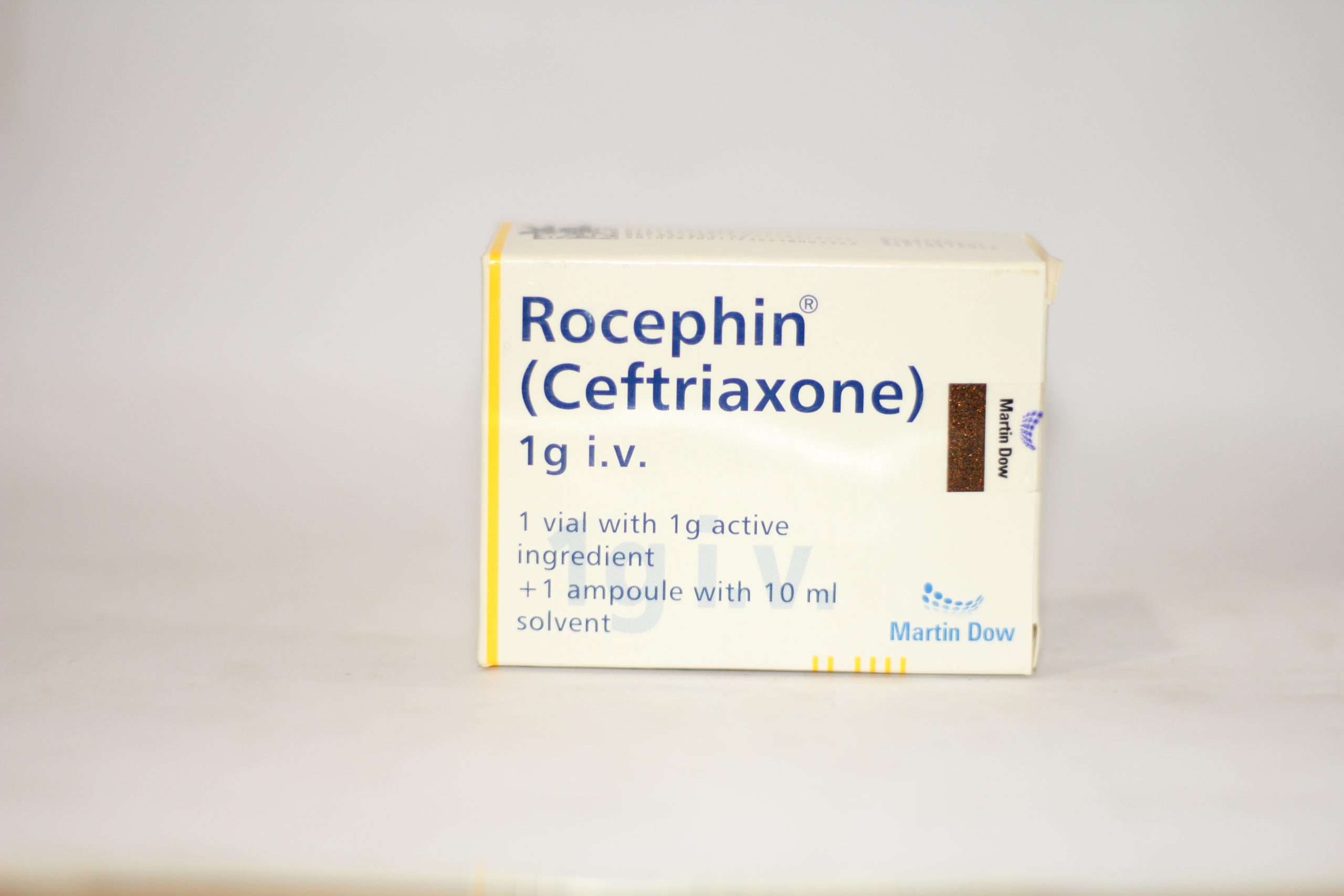
It turns out, the question "why was Rocephin discontinued" actually points to a common misconception. Rocephin, which is the brand name for a medicine called ceftriaxone, is actually still very much in use today. It's a widely used antibiotic, and it helps people with all sorts of infections. So, if you were wondering about its status, you can put those worries to rest. But, that doesn't mean we can't explore the deeper reasons why people might think a drug like this would disappear, and what truly makes a medicine leave the shelves.
- Rocephin: The Antibiotic That Persists
- Typical Reasons a Drug Might Be Discontinued
- The Process of Drug Development and Monitoring
- The Human Impact of Drug Availability
- Frequently Asked Questions (FAQs)
- Conclusion: Understanding Drug Status
Rocephin: The Antibiotic That Persists
It's interesting, isn't it, how certain ideas can take hold, even if they aren't quite accurate? The idea that Rocephin, or ceftriaxone, was discontinued is one such thought. Perhaps it comes from seeing different brand names, or maybe just a general feeling that things change over time. But, in fact, this important medicine continues to play a big part in helping people get better. It's really quite common for people to wonder about things like this, just like asking why a certain word sounds a bit strange in one situation but perfectly normal in another, you know?
What Is Rocephin (Ceftriaxone)?
Rocephin is, actually, a very powerful antibiotic. It's a member of a group of medicines called cephalosporins. Doctors often use it to treat a wide range of bacterial infections, like pneumonia, skin infections, and even certain types of meningitis. It works by stopping bacteria from building their cell walls, which basically kills them off. So, it's pretty effective at what it does, and it has been for a good long while.
This medicine, ceftriaxone, is typically given as an injection, either into a muscle or a vein. It's not something you usually take as a pill. That might be part of why some people don't see it as often as other medications they might take at home. It's more of a hospital or clinic kind of medicine, you could say. It's often used when infections are quite serious, or when other antibiotics haven't worked out, so it's a bit of a go-to for many healthcare providers, in some respects.
Why the Misconception About Discontinuation?
So, why do people ask, "why was Rocephin discontinued?" There could be a few reasons, really. One thought is that sometimes a specific brand name might become less common, even if the generic version of the medicine is still widely available. For instance, Rocephin is the brand name, but ceftriaxone is the generic name. Doctors and pharmacies often use the generic name more often these days, which can make it seem like the brand name product has vanished. That's a pretty common thing to happen with medicines, as a matter of fact.
Another reason might be that there are so many new antibiotics always coming out. With new options, people might assume older ones are being phased out. But that's not always the case. Some older medicines, like ceftriaxone, are still very effective and important, especially when we consider things like antibiotic resistance. So, it's not just about what's new, but what still works reliably, you know?
Also, sometimes there are temporary shortages of medications due to manufacturing issues or supply chain hiccups. These can make it seem like a drug is gone for good, when it's just a temporary problem. These kinds of situations can cause a bit of worry and confusion for people, naturally. It's like when you can't find your favorite cereal on the shelf for a week or two, and you wonder if they stopped making it.
Typical Reasons a Drug Might Be Discontinued
While Rocephin is still here, it's worth thinking about what actually makes a drug leave the market. It's not a simple decision, and it usually involves a lot of different factors. Understanding these reasons can help us appreciate the care that goes into making sure the medicines we use are safe and helpful. This is, you know, a pretty important thing to consider when we talk about healthcare.
Safety Concerns and Unwanted Effects
One of the biggest reasons a drug might be pulled from the market is if serious safety concerns come up. Even after a medicine is approved and being used by many people, new or more severe unwanted effects might be discovered. If these risks outweigh the benefits of the medicine, then health authorities and the drug makers might decide it's too risky to keep it available. This is, quite frankly, the most serious reason for a drug to go away.
Sometimes, these issues aren't obvious during the initial testing phases because those studies involve a smaller number of people. When a drug is used by millions, rare but serious problems can become more apparent. For example, a medicine might cause a very rare heart problem in one out of a hundred thousand people. That's something you might not see until it's out in the wider population. So, monitoring medicines after they are approved is really, really important.
Lack of Effectiveness
Another reason a drug might be discontinued is if it turns out it's just not working as well as everyone thought it would. Maybe the initial studies looked promising, but in the real world, it doesn't help patients as much as expected. Or, perhaps, over time, a disease changes, or the bugs it's fighting become resistant, making the medicine less useful. This is particularly true for antibiotics, where bacteria can evolve and learn to fight off the drugs. It's a constant challenge, you know?
If a medicine isn't really making a difference for patients, then there's not much point in keeping it around, especially if there are other, more effective options. The goal, after all, is to help people get better, and if a medicine isn't doing that job, then it's time to move on. That's just common sense, in a way.
New and Improved Alternatives
Sometimes, a drug is discontinued not because it's bad, but because something better comes along. Science is always moving forward, and researchers are always finding new ways to treat illnesses. A newer medicine might be more effective, have fewer unwanted effects, or be easier for patients to take. When a clearly superior option becomes available, it can make an older drug less necessary. This is, you know, a positive kind of discontinuation, in some respects.
For instance, if a new antibiotic comes out that can treat the same infections as Rocephin but with fewer doses or less pain from the injection, then doctors might start using that new one more often. The older drug might then slowly fade out of use, not because it was bad, but because it was simply outshone. It's a bit like older technology being replaced by newer, more convenient versions.
Manufacturing and Supply Chain Challenges
Making medicines is a very complex process. It involves getting the right ingredients, using specialized equipment, and following very strict quality rules. Sometimes, a drug might be discontinued because the company can no longer make it reliably or affordably. This could be due to problems getting the raw materials, issues with the factories, or even just the cost of production becoming too high. These are practical, behind-the-scenes reasons, but they can have a big impact. It's like, you know, if a car company can't get the parts they need anymore.
These kinds of issues can lead to shortages, which can be very frustrating for patients and healthcare providers. If a shortage becomes long-term or impossible to fix, the company might decide to stop making the drug altogether. This is a business decision, but it's one that affects real people. So, it's pretty serious when these things happen, actually.
Business and Market Considerations
Drug companies are, you know, businesses, and sometimes their decisions are based on economic factors. A medicine might be discontinued if it's no longer profitable to make it. This could happen if the patent expires and generic versions flood the market, driving down prices. Or, if the demand for the drug is simply too low, it might not be worth the cost of production and distribution. It's a bit like any other product, really; if it doesn't sell, companies eventually stop making it.
This doesn't mean the drug is unsafe or ineffective, just that it's not financially viable for the company to keep producing it. In such cases, another company might pick up the manufacturing of the generic version, so the medicine doesn't disappear entirely. But the original brand might be gone. This is a common part of the life cycle of many products, including medicines, so it's not entirely surprising, in a way.
Regulatory Decisions
Government health agencies, like the Food and Drug Administration (FDA) in the United States, play a very big role in deciding which drugs are available. They constantly monitor medicines for safety and effectiveness. If they find that a drug no longer meets their standards, they can order a recall or even force a company to stop selling it. This is a very serious step, and it's usually taken only when there are significant risks to public health. They are, essentially, the big cheese when it comes to drug approval and monitoring, if you get what I mean.
These agencies also have to approve any changes to how a drug is made or what it's used for. If a company can't meet the regulatory requirements, that can also lead to a drug being pulled. Their job is to protect people, so they take these decisions very, very seriously. It's a complex system, but it's there for a good reason, you know?
The Process of Drug Development and Monitoring
It's fascinating, really, how much goes into getting a medicine from an idea to something that helps people. The journey of a drug is long and full of careful steps. First, there's a lot of research and testing in labs. Then, if things look promising, it moves to human trials, which happen in different phases, each one getting bigger and more detailed. This whole process can take many, many years, actually.
Even after a drug is approved and out in the world, the monitoring doesn't stop. Health professionals and patients are encouraged to report any unwanted effects they experience. This information is collected and reviewed by regulatory bodies. This ongoing watch helps catch any problems that might not have shown up in earlier studies. It's a system designed to keep us safe, more or less, and it's always working, even if we don't always see it.
This continuous oversight is why, even if a drug like Rocephin has been around for decades, its safety and effectiveness are still being watched. It's not a "set it and forget it" kind of situation. This constant vigilance is, you know, a very important part of modern medicine. It helps ensure that if a reason were to arise for a drug to be discontinued, it would be caught and acted upon.
The Human Impact of Drug Availability
When a medicine is discontinued, or even when there's just a rumor about it, it can cause a lot of worry for people. Patients who rely on a specific drug might suddenly feel uncertain about their treatment. Doctors have to figure out alternative ways to help their patients, which can be tricky. It's not just about the science; it's about people's lives and their well-being. This is where the human side of medicine really comes into play, you know?
For example, if a drug that treats a rare condition were to disappear, finding a suitable replacement could be very hard, and sometimes there might not be one. This highlights why it's so important for accurate information to be available about drug status and why health authorities try their best to manage transitions smoothly if a drug truly needs to be pulled. It's a bit like if you suddenly couldn't get a specific part for something you really needed; it would cause a lot of trouble.
So, while the question "why was Rocephin discontinued" might come from a place of confusion, it also shows how much people care about the availability of their medicines. It underscores the need for clear communication from healthcare providers and reliable sources. Knowing the real story helps everyone feel a bit more secure about their health choices, which is pretty important, honestly.
Frequently Asked Questions (FAQs)
People often have questions about medicines, and that's a good thing. Here are a few common ones that pop up, especially around topics like drug availability:
Is Rocephin still used in hospitals today?
Yes, absolutely! Rocephin (ceftriaxone) is very much still a widely used and important antibiotic in hospitals and clinics all over the world. Doctors use it to treat a whole bunch of serious bacterial infections. It's a bit of a staple, you know, for many different conditions.
What is ceftriaxone used for?
Ceftriaxone, which is the generic name for Rocephin, is used to treat a broad range of bacterial infections. This includes things like pneumonia, urinary tract infections, skin and soft tissue infections, bone and joint infections, and even certain types of meningitis and gonorrhea. It's a versatile medicine, pretty much.
How do I know if a medication has been discontinued?
The best way to find out if a medication has been discontinued is to ask your doctor or pharmacist. They have access to the most up-to-date information. You can also check official health authority websites, like the FDA's drug shortages and discontinuations database, which is a pretty reliable source. This helps you get the real facts, as a matter of fact.
Conclusion: Understanding Drug Status
So, while the question "why was Rocephin discontinued" comes up quite a bit, it's good to know that Rocephin, or ceftriaxone, is still a vital part of our medical toolkit. It's fascinating how a simple question can lead us to explore the many reasons why medicines might, or might not, leave the market. These reasons are complex, ranging from safety and effectiveness to manufacturing and market forces. It's never just one thing, you know?
Understanding these different factors helps us appreciate the careful process that ensures the medicines we rely on are both safe and helpful. It also reminds us how important it is to get accurate information, especially about something as personal as our health. For more general information on medication use and safety, you could always check out resources like the World Health Organization's patient safety initiatives. You can learn more about general health topics on our site, and find more details about common medical questions here. Knowing the facts helps everyone feel more confident about their health choices, which is a pretty good thing, I think.


Detail Author:
- Name : Prof. Tomas Hegmann
- Username : sienna61
- Email : oconnell.damon@hotmail.com
- Birthdate : 1972-05-15
- Address : 21548 DuBuque Harbors Apt. 349 Ullrichberg, MO 92027-5928
- Phone : +16299220678
- Company : Deckow Ltd
- Job : Brake Machine Setter
- Bio : Praesentium reiciendis dolorem est aspernatur sequi neque ratione. Et suscipit eos hic aperiam. Sit occaecati nihil at. Quo non adipisci pariatur quae laboriosam est error dicta.
Socials
instagram:
- url : https://instagram.com/manuel_official
- username : manuel_official
- bio : Enim ipsa quis qui esse dicta. Ipsum sequi et odit et voluptas exercitationem.
- followers : 5313
- following : 1951
tiktok:
- url : https://tiktok.com/@hahnm
- username : hahnm
- bio : Error earum eum suscipit aut voluptatem nam. Et facilis nemo beatae provident.
- followers : 4438
- following : 292
facebook:
- url : https://facebook.com/hahn2006
- username : hahn2006
- bio : Facilis quidem dolorem voluptate animi commodi earum.
- followers : 4914
- following : 2188